
Why Paints, Coatings, and Adhesives Companies Add Zinc Oxide to Their Products
Zinc oxide, or ZnO as it’s more commonly referred to, is an insidious powerhouse in surface technologies today. Formulators incorporate it into paints, coatings, and adhesives because a single, well-selected grade can provide ultraviolet protection, corrosion resistance, antimicrobial durability, mechanical strengthening, optical stability, rheology control, and enhanced adhesion. That range of function is rare and affects markets where failure manifests as chalky siding, rusty handrails, peeling floor finishes, or delaminated laminates.
UV screening and photostability
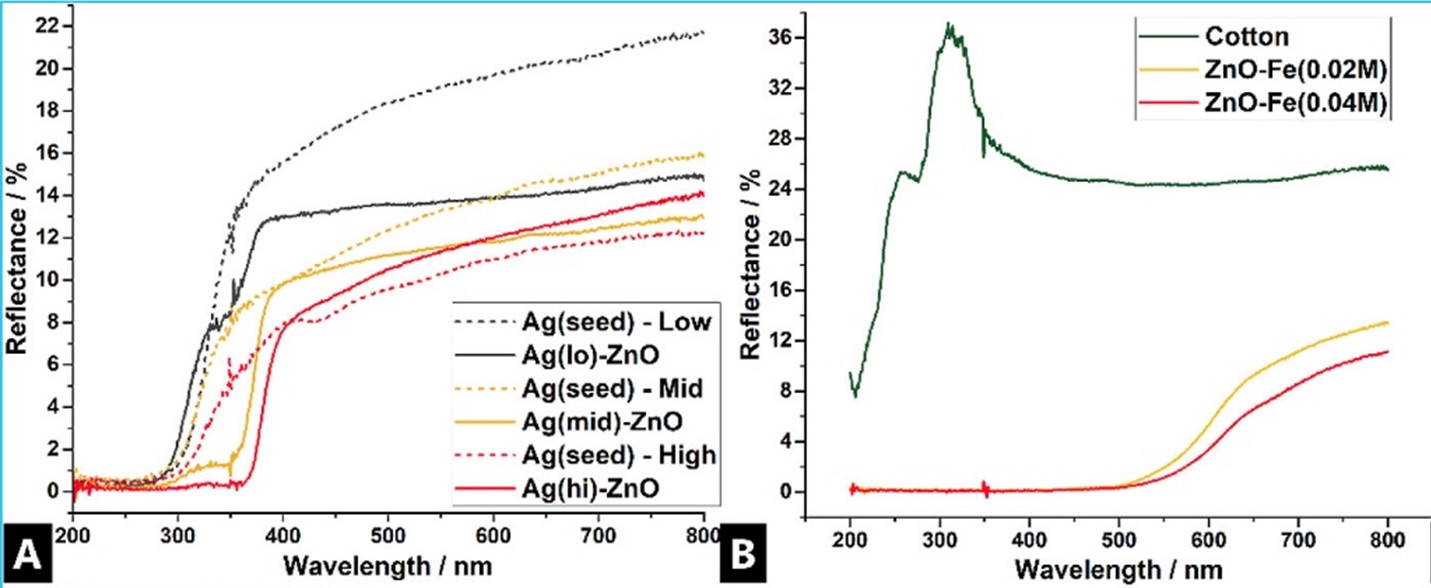
The wurtzite crystal structure of zinc oxide has a broad band gap that interacts strongly with ultraviolet radiation in the wavelength region of approximately 280 to 400 nanometers. When dispersed in a film, its particles absorb and scatter energy before photons interact with the organic binder. Exterior architectural coatings chalk more slowly; transparent wood coatings retain lignin; marine and automotive coatings are resistant to yellowing and cracking.

Anticorrosion and barrier performance
Where metals are present, zinc oxide provides a second line of defense. Physically, ZnO densifies coatings and enhances tortuosity, extending the practical pathway for water, oxygen, and ions. Chemically, it can react with water to form basic zinc salts; the precipitates seal microdefects and strengthen the barrier. In epoxy and polyurethane primers, ZnO’s polar surface promotes wetting and adhesion on steel and galvanized substrates and reduces underfilm corrosion in salt spray and humidity testing. It is not a sacrificial pigment like metallic zinc dust, but it provides valuable time to red rust and reduces corrosion blisters after scribing.
Antimicrobial and mildew resistance
Part of the zinc released on the coating surface interferes with membranes and metabolic processes in microorganisms.
That long-lasting, inorganic mechanism of action renders ZnO effective in bathrooms, kitchens, hospitals, food plants, and wet basements, where mildew and odor are ongoing issues. Water-borne adhesives and sealants also improve in storage; a low dose of ZnO prevents slime-formers and keeps tanks and hoses clean without relying solely on volatile preservatives.
Mechanical reinforcement and toughness
Zinc oxide is an active filler. Its high-energy, polar surface interacts with epoxy, acrylic, polyurethane, nitrile, and chloroprene functionality, increasing cohesive strength. Films are harder, more abrasion-resistant, and more block-resistant. Pressure-sensitive and structural adhesives exhibit increased modulus and creep resistance, which maintains bondline geometry under temperature and load. Since ZnO can be used in small particle sizes, these advantages are realized without excessive brittleness or opacity.
Rheology control and application
Properly dispersed ZnO delivers gentle thixotropy. During brushing, rolling, or spraying, viscosity drops to aid leveling; after application, it rebounds, resisting sag, edge slumping, and pigment settling. Surface-treated grades—silanes, stearates, or polymer coatings—let chemists tune compatibility with binders and surfactants, reducing flocculation with titanium dioxide and organic colorants. The payoff is smoother application, sharper cut-ins, and consistent film build on verticals.
Optical Management and Tint Stability
It has no equal in brightness, though TiO₂ can cause color defects and photocatalysis. Mixing in a blend of ZnO will compensate for undertones, improve hiding in the near-UV, and extend the longevity of light colors when exposed to UV radiation. “Invisible” UV protection is offered by nano-scale zinc oxide particles when used in clear coats, causing neither opacity nor loss of image clarity; it also protects wood, plastics, and printed images from UV degradation beneath brightly coated surfaces.
Thermal and chemical durability
With a melting point around 1975°C and its amphoteric properties, zinc oxide is resistant to cure conditions and limits the action of acids or bases that can embrittle other films. Heat-resistant, coil-coated products use ZnO to maintain gloss and flexibility through repeated heating and cooling cycles. Aggressive environments in refineries, fertilizer plants, and sewage treatment operations enable ZnO to maintain its barrier properties despite exposure to cleaning agents, fuels, and salt residues that seek to disrupt it.
Adhesion Promotion and Cure Kinetics
The zinc centers provide Lewis acid functionality that coordinates well with either an oxygen or a nitrogen donor, thereby catalyzing interfacial cross-linking. The faster gelation near the substrate will result in shorter production times in the production lines, along with improvements in lap-shear strength and peel adhesion. ZnO enhances the condensation process in moisture-cure polyurethane or silyl-terminated polymers.
Table 1: Adhesion Promotion and Cure Kinetics of ZnO in Coatings and Adhesives
| Feature/Benefit | Mechanism | Outcome |
| Catalysis of interfacial cross-linking | Zn²⁺ acts as a Lewis acid; it coordinates with oxygen or nitrogen donors | Faster gelation near substrate; improved productivity |
| Improved adhesion | Chemical interaction between ZnO and polymer matrix | Increased lap-shear and peel strength |
| Supports moisture-curing chemistry | Participates in the condensation of silane- or polyurethane-based systems | Enhanced film durability, especially in humid conditions |
| Maintains film integrity under curing stress | Stabilizes bonding interactions across temperature ranges | Improved bondline geometry retention and mechanical reliability |
Sustainability and compliance advantages
The compound improves indoor air quality by reducing VOCs, since it is a low-emitting, low-odor agent. The enhanced durability also leads to lower repainting rates, resulting in lower emissions over the service life. The use of recycled zinc has environmental product declarations from various suppliers, ensuring it can support company sustainability requirements. The substitution process for preservatives, UV stabilizers, or UV absorbers also improves health and safety reporting by simplifying chemical requirements.
Specification, processing, and quality control
The benefits lie in the details. Particle size distribution affects optical properties and reinforcement, and submicron particles provide maximum UV protection and smoothness, while particles only slightly larger enable better viscosity and cost-effectiveness. Specific surface affects the requirements for thixotrypsin and dispersants. Surface chemistry distinguishes water-borne from solvent-borne formulations, ensuring that ZnO does not react adversely with drier or catalytic components. The absence of impurities, notably lead, cadmium, and chloride, remains essential for compliance and will not catalyze coloring either. On the manufacturing side, premixes using high shear enable wetting of ZnO particles, while specific dispersers prevent agglomerations by controlling the addition rate and temperature.
Economics and Proof
Despite the potential for higher cost compared to passive additives, the actual cost savings achieved by using ZnO are usually offset by its benefits, such as longer life, reduced callbacks, reduced preservatives, improved production rate, and faster cure time. Standard tests, such as QUV, xenon, salt spray, humidity, abrasion, impact, bend, and lap shear, can also express the benefit.
Conclusion
The reasons formulations ‘ suppliers add zinc oxide lie in its multifunctionality. It protects against the sun’s rays, inhibits corrosion, reduces microbial growth, increases the strength of thin films, enhances ease of application, adjusts colors, protects against heat and chemical agents, enhances adhesion, and, if needed, simultaneously supports sustainable objectives. Zinc oxide can perform multiple functions when its attributes, such as particle size, surface treatments, and purity, are optimized.

